|
Our MACRA Programs
Organizations
|
|
|
|||||||||||||||||
|
Our
QCDR-HISP
Submission System
We offer a complete CMS Submission publishing system for your organization.
Our extensive experience will expedite QCDR submissions for your providers.
> See details on our CMS Submission
Electronic Publishing.
More..."
> We merge multiple data sources specific to
your metrics.
More... > We save your time with our CST (CMS Submission Template)
> The CST
provide
a detailed schema and
methods for automated review at discrete stages of the report generation
process to meet the stringent CMS data requirements.
More...
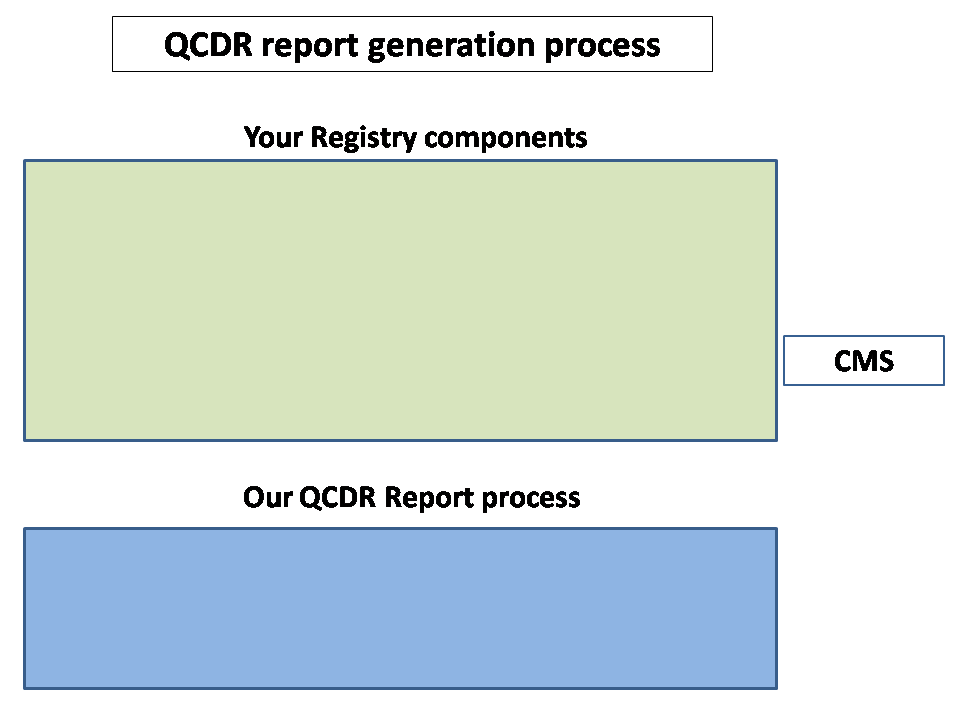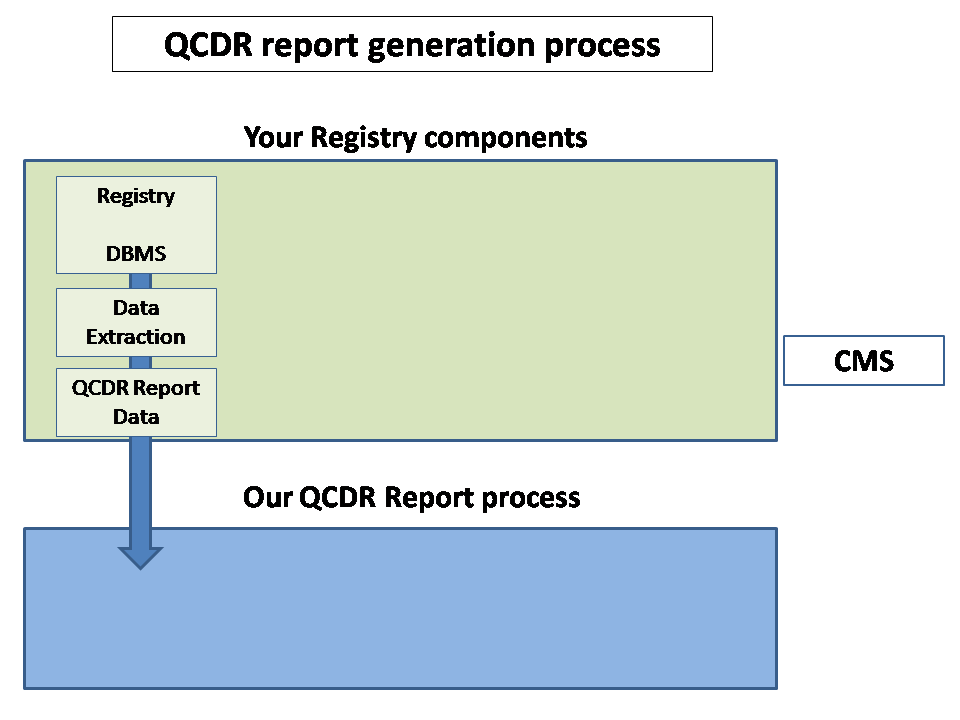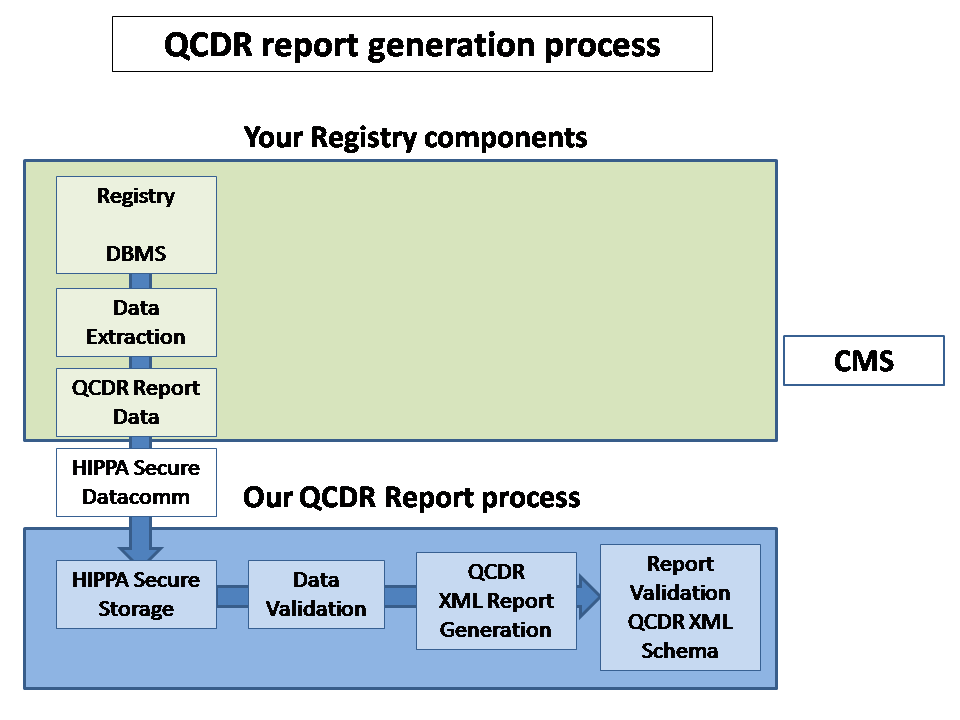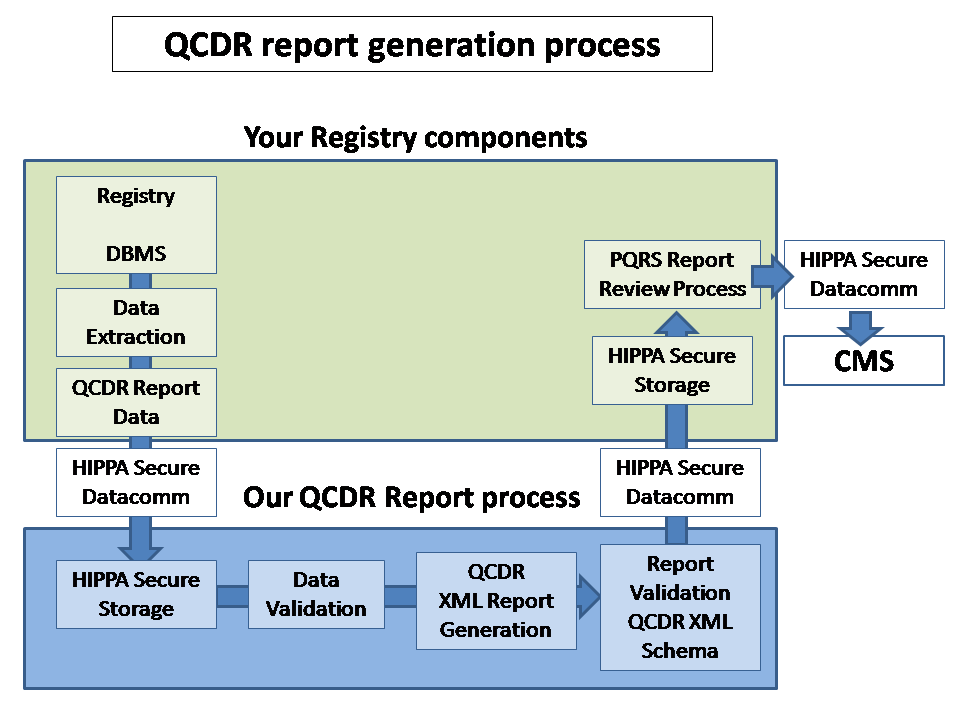
> We use valuable clinician time
judiciously by isolating elements of the CMS electronic report the require
clinical judgment. More... > Our HIPAA datacomm securely exchanges clinical data and submissions. More...
CMS Submission process - without CUHSM.ORG: You will find precious clinician time wasted on re-inventing design, implementation and the intensive review processes for the massive amounts of clinical data inherent in CMS submissions.
CMS Submission process - with CUHSM.ORG: Your expedited QCDR submissions will meet tight CMS deadlines and be applied to assess and plan improvement cycles of your relevant healthcare processes.
|
.